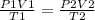Chemistry, 21.06.2019 17:00 Luzperez09
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1


Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1
You know the right answer?
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 29...
Questions

History, 13.07.2020 20:01

Geography, 13.07.2020 20:01

Mathematics, 13.07.2020 20:01


Mathematics, 13.07.2020 20:01

Health, 13.07.2020 20:01

Mathematics, 13.07.2020 20:01

Spanish, 13.07.2020 20:01






Mathematics, 13.07.2020 20:01


Mathematics, 13.07.2020 20:01




Mathematics, 13.07.2020 20:01