

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
Chlorine (cl) has an atomic number of 17. it often forms an ion by gaining 1 electron. what would it...
Questions


Mathematics, 24.06.2019 16:00

Mathematics, 24.06.2019 16:00

Mathematics, 24.06.2019 16:00

Social Studies, 24.06.2019 16:00


Biology, 24.06.2019 16:00






History, 24.06.2019 16:00

Health, 24.06.2019 16:00


Computers and Technology, 24.06.2019 16:00



Computers and Technology, 24.06.2019 16:00

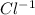 .
.

