
Chemistry, 22.06.2019 12:10 purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrati...
Questions

History, 05.05.2020 15:10

Mathematics, 05.05.2020 15:10

Mathematics, 05.05.2020 15:10


Mathematics, 05.05.2020 15:10


Mathematics, 05.05.2020 15:10


Mathematics, 05.05.2020 15:10

English, 05.05.2020 15:10


Biology, 05.05.2020 15:10

Social Studies, 05.05.2020 15:10

History, 05.05.2020 15:10

History, 05.05.2020 15:10


Biology, 05.05.2020 15:10


Geography, 05.05.2020 15:10

![[NO]=0.011mol/L](/tpl/images/0004/0244/27865.png)
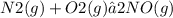
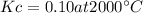
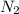
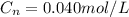
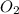
![KC=\frac{(NO)^2}{[N_2][O_2]}](/tpl/images/0004/0244/d7dda.png)
![[NO]^2=0.10*0.040*0.040](/tpl/images/0004/0244/6785f.png)
![[NO]^2=1.6*10^{-4}](/tpl/images/0004/0244/f08ee.png)
![\left[\begin{array}{cccc}&N2&O2&NO\\I&0.04&0.04&0\\C&-x&-x&+2x\\E&0.04-x&0.04-x&2x\end{array}\right]](/tpl/images/0004/0244/697dd.png)


