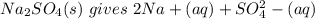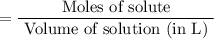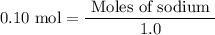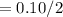Chemistry, 22.06.2019 12:30 kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 ar...
Questions


History, 20.05.2021 02:20

Mathematics, 20.05.2021 02:20

Mathematics, 20.05.2021 02:20

English, 20.05.2021 02:20



Computers and Technology, 20.05.2021 02:20


Mathematics, 20.05.2021 02:20

Mathematics, 20.05.2021 02:20

English, 20.05.2021 02:20


Mathematics, 20.05.2021 02:20

Physics, 20.05.2021 02:20


Mathematics, 20.05.2021 02:20

French, 20.05.2021 02:20


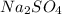 is 0.05 moles.
is 0.05 moles.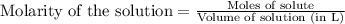
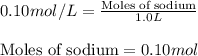
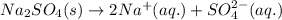
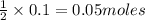 of sodium sulfate.
of sodium sulfate.