Chemistry, 05.02.2020 00:02 mercydiaz84
(a) write the balanced neutralization reaction that occurs between h2so4 and koh in aqueous solution. phases are optional. (b) suppose 0.750 l of 0.480 m h2so4 is mixed with 0.700 l of 0.290 m koh. what concentration of sulfuric acid remains after neutralization?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1
You know the right answer?
(a) write the balanced neutralization reaction that occurs between h2so4 and koh in aqueous solution...
Questions

Mathematics, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

English, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Social Studies, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Health, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Spanish, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01

Mathematics, 17.09.2020 15:01


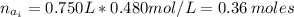
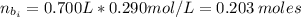
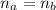
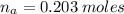
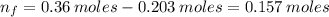
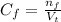
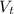 : is the total volume = (0.750 + 0.700) L = 1.45 L
: is the total volume = (0.750 + 0.700) L = 1.45 L