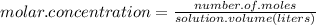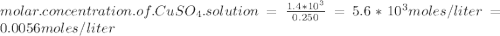One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: fe(s) + cuso4(aq) → cu(s) + feso4(aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a 250.ml copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of 89.mg. calculate the original concentration of copper(ii) sulfate in the sample. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1


Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which con...
Questions


Mathematics, 31.07.2021 01:00





Mathematics, 31.07.2021 01:00

Mathematics, 31.07.2021 01:00




History, 31.07.2021 01:00








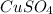 solution = 0.0056 moles / liter
solution = 0.0056 moles / liter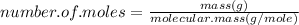
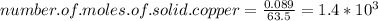
 moles of Cu equals to
moles of Cu equals to