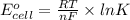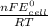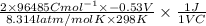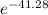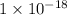What is the value of the equilibrium constant at 25 oc for the reaction between the pair: i2(s) and br-(aq) give your answer using e-notation with no decimal places (e. g., 2 x 10-2 would be 2e-2; and 2.12 x 10-2 would also be 2e- use the reduction potentials for i2(s) is 0.535 v and for br2(l) is +1.065 v.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1


Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

You know the right answer?
What is the value of the equilibrium constant at 25 oc for the reaction between the pair: i2(s) and...
Questions


Physics, 06.12.2019 03:31

Mathematics, 06.12.2019 03:31



Mathematics, 06.12.2019 03:31


Mathematics, 06.12.2019 03:31


Mathematics, 06.12.2019 03:31


Mathematics, 06.12.2019 03:31


Mathematics, 06.12.2019 03:31


History, 06.12.2019 03:31

Mathematics, 06.12.2019 03:31


Geography, 06.12.2019 03:31

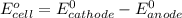
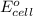 and K is as follows.
and K is as follows.