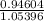Chemistry, 30.08.2019 17:20 brisamauro27
Calculate the change in ph when 71.0 ml of a 0.760 m solution of naoh is added to 1.00 l of a solution that is 1.0o m in sodium acetate and 1.00 m in acetic acid.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Calculate the change in ph when 71.0 ml of a 0.760 m solution of naoh is added to 1.00 l of a soluti...
Questions





English, 15.07.2019 17:30

Spanish, 15.07.2019 17:30

Spanish, 15.07.2019 17:30


Computers and Technology, 15.07.2019 17:30

English, 15.07.2019 17:30

Mathematics, 15.07.2019 17:30

Biology, 15.07.2019 17:30

Social Studies, 15.07.2019 17:30

Mathematics, 15.07.2019 17:30


History, 15.07.2019 17:30

Chemistry, 15.07.2019 17:30



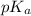 value of acetic acid is 4.74. And, relation between pH and
value of acetic acid is 4.74. And, relation between pH and ![\frac{[CH_{3}COOH]}{[CH_{3}COONa]}](/tpl/images/0212/4640/b577a.png)
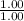
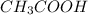 = moles of
= moles of 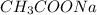
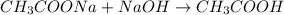
![\frac{[CH_{3}COONa]}{[CH_{3}COOH]}](/tpl/images/0212/4640/ccc54.png)