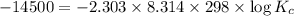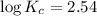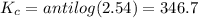Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
Areaction has a standard free-energy change of -14.50 kj mol(-3.466 kcal mol). calculate the equilib...
Questions

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Physics, 14.09.2020 15:01

English, 14.09.2020 15:01

History, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

History, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Biology, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

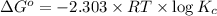
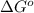 = standard Gibb's free energy change = -14.50kJ/mol =14500 J/mol
= standard Gibb's free energy change = -14.50kJ/mol =14500 J/mol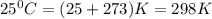
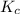 = equilibrium constant = ?
= equilibrium constant = ?