
Chemistry, 22.09.2019 03:20 chelseayazzie16
Asolution of heparin sodium, an anticoagulant for blood, contains 1.8 g of heparin sodium dissolved to make a final volume of 15 ml of solution. what is the mass/volume percent concentration of this solution?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
Asolution of heparin sodium, an anticoagulant for blood, contains 1.8 g of heparin sodium dissolved...
Questions



Mathematics, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30

History, 07.10.2019 22:30


English, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30

English, 07.10.2019 22:30


Mathematics, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30


Biology, 07.10.2019 22:30

Computers and Technology, 07.10.2019 22:30




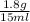 x 100 ml = 12 % m/v
x 100 ml = 12 % m/v

