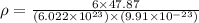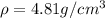Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
Titanium has an hcp unit cell for which the ratio of the lattice parameters cais 1.58. if the radius...
Questions

Mathematics, 14.12.2019 11:31




Mathematics, 14.12.2019 11:31

English, 14.12.2019 11:31

History, 14.12.2019 11:31


Mathematics, 14.12.2019 11:31

Mathematics, 14.12.2019 11:31


Mathematics, 14.12.2019 11:31




Mathematics, 14.12.2019 11:31


Mathematics, 14.12.2019 11:31

History, 14.12.2019 11:31

Mathematics, 14.12.2019 11:31

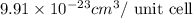
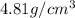
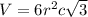
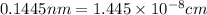
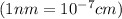
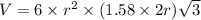
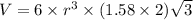
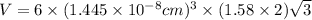
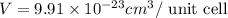
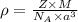
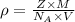 ..........(1)
..........(1)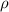 = density of Ti = ?
= density of Ti = ?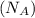 = Avogadro's number =
= Avogadro's number = 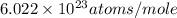
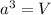 = volume of unit cell =
= volume of unit cell =