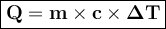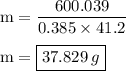Chemistry, 27.09.2019 17:30 Annaborden02
Ablock of copper of unknown mass has an initial temperature of 65.4 ∘c. the copper is immersed in a beaker containing 95.7 g of water at 22.7 ∘c. when the two substances reach thermal equilibrium, the final temperature is 24.2 ∘c. what is the mass of the copper block? express your answer in grams to three significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
Ablock of copper of unknown mass has an initial temperature of 65.4 ∘c. the copper is immersed in a...
Questions


Arts, 28.10.2020 18:10


World Languages, 28.10.2020 18:10


Mathematics, 28.10.2020 18:10

Mathematics, 28.10.2020 18:10


Spanish, 28.10.2020 18:10




Mathematics, 28.10.2020 18:10

Biology, 28.10.2020 18:10



Mathematics, 28.10.2020 18:10

Geography, 28.10.2020 18:10