
Three isotopes of argon occur in nature – 36 18ar, 38 18ar, 40 18ar. calculate the average atomic mass of argon to two decimal places, given the following relative atomic masses and the abundances of each of the isotopes: argon36 (35.97 amu; 0.337%), argon-38 (37.96 amu; 0.063%), argon-40 (39.96 amu; 99.600%).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1


Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
Three isotopes of argon occur in nature – 36 18ar, 38 18ar, 40 18ar. calculate the average atomic ma...
Questions

Mathematics, 27.09.2020 15:01

Mathematics, 27.09.2020 15:01


Mathematics, 27.09.2020 15:01

Mathematics, 27.09.2020 15:01


Chemistry, 27.09.2020 15:01



Mathematics, 27.09.2020 15:01



Mathematics, 27.09.2020 15:01




Chemistry, 27.09.2020 15:01

Computers and Technology, 27.09.2020 15:01


History, 27.09.2020 15:01

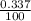 x 35.97) + (
x 35.97) + (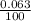 x 37.96) +(
x 37.96) +(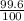 x 39.96)
x 39.96)

