
Chemistry, 17.10.2019 05:00 trinityanne1738
At equilibrium, the concentrations in this system were found to be [n2]=[o2]=0.200 m and [no]=0.600 m. n2(g)+o2(g)↽−−⇀2no(g) if more no is added, bringing its concentration to 0.900 m, what will the final concentration of no be after equilibrium is re‑established?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1
You know the right answer?
At equilibrium, the concentrations in this system were found to be [n2]=[o2]=0.200 m and [no]=0.600...
Questions


Biology, 15.10.2019 01:00




Mathematics, 15.10.2019 01:00



Mathematics, 15.10.2019 01:00

Biology, 15.10.2019 01:00

English, 15.10.2019 01:00

Biology, 15.10.2019 01:00


Biology, 15.10.2019 01:00


Health, 15.10.2019 01:00

Mathematics, 15.10.2019 01:00

Biology, 15.10.2019 01:00

Mathematics, 15.10.2019 01:00

Mathematics, 15.10.2019 01:00

![K_{eq}=\frac{[NO]^2_{eq}}{[N_2]_{eq}[O_2]_{eq}} \\K_{eq}=\frac{0.6^2}{0.2*0.2}\\ K_{eq}=9](/tpl/images/0327/3782/fd314.png)
![9=\frac{[NO]^2_{eq}}{[N_2]_{eq}[O_2]_{eq}}\\9=\frac{[0.9+2x]^2}{[0.2-x][0.2-x]}](/tpl/images/0327/3782/c867b.png)
![\frac{1}{9} =\frac{[N_2]_{eq}[O_2]_{eq}}{[NO]^2_{eq}}\\\frac{1}{9} =\frac{[0.2+x][0.2+x]}{[0.9-2x]^2}](/tpl/images/0327/3782/3755f.png)
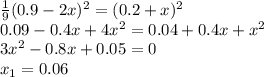
![[NO]_{eq}=0.9-0.06=0.84M](/tpl/images/0327/3782/19d1e.png)


