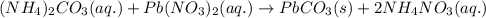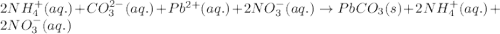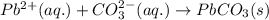(c) ammonium carbonate + lead nitrate ? step-by-step solution step 1 of 3 v step 2 of 3 v step 3 of 3 ^ c) the ions formed in solution are nh4+, co2, pb2+ and no3, combine and form soluble compound nh. no, so the ions nh,* and no3 remain in solution, whereas pb2+ and co2-form insoluble compound pbco; total ionic equation is: 2 nh,+ (aq) + c0,- (aq) + pb* (aq) + 2 n0,- (aq) → 2 nh,+ (aq) + 2 no,- (aq) + pbco () net ionic equation is: pb2+ (aq) + co2-(aq) → pbco(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
(c) ammonium carbonate + lead nitrate ? step-by-step solution step 1 of 3 v step 2 of 3 v step 3 of...
Questions

Biology, 13.07.2019 07:00

Health, 13.07.2019 07:00

History, 13.07.2019 07:00

Mathematics, 13.07.2019 07:00

Mathematics, 13.07.2019 07:00



Mathematics, 13.07.2019 07:00

History, 13.07.2019 07:00


Mathematics, 13.07.2019 07:00


Mathematics, 13.07.2019 07:00


Mathematics, 13.07.2019 07:00

Mathematics, 13.07.2019 07:00


Mathematics, 13.07.2019 07:00

Social Studies, 13.07.2019 07:00

Mathematics, 13.07.2019 07:00