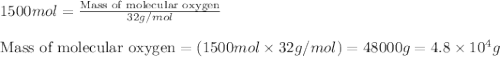Chemistry, 18.10.2019 03:30 2021danaejalang
35. ammonium perchlorate, nh co., is a common fuel component of solid fuel rockets. if 75% of the oxygen in nh. co. was converted to molecular oxygen, how many grams of molecular oxygen would be produced from 117.5 kg ammonium perchlorate? (atomic weights in amu: n = 14.01, ci = 35.45, 0 = 16.00, h = 1.008) a. 4.8 x 10^g 02 b. 6.4 x 10^g 02 c. 2.2 x 10^g 02 d. 2.4 x 10^g 02

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1

You know the right answer?
35. ammonium perchlorate, nh co., is a common fuel component of solid fuel rockets. if 75% of the ox...
Questions

French, 21.01.2021 20:40




Health, 21.01.2021 20:40


English, 21.01.2021 20:40


English, 21.01.2021 20:40


Mathematics, 21.01.2021 20:40

Mathematics, 21.01.2021 20:40


Physics, 21.01.2021 20:40

Mathematics, 21.01.2021 20:40

Mathematics, 21.01.2021 20:40

English, 21.01.2021 20:40



Mathematics, 21.01.2021 20:40

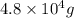
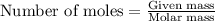 .....(1)
.....(1)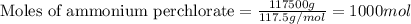
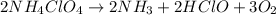
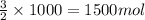 of molecular oxygen
of molecular oxygen