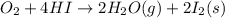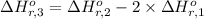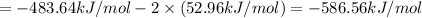Chemistry, 18.10.2019 03:30 miya257916
Calculate the standard enthalpy of the 3rd reaction using the given data: h2(g) +126) +2 hio) a, hº = +52.96 kj/mol 2 h2(g) + o2(g) + 2 h209) a, hº = - 483.64 kj/mol 4 hi) + o2(g) +2 12(8) + 2 h206) a, hº =? a. h=-589.5619/mol calculate the internal energy change for reaction (3) in the previous problem.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1
You know the right answer?
Calculate the standard enthalpy of the 3rd reaction using the given data: h2(g) +126) +2 hio) a, hº...
Questions







Engineering, 15.12.2020 23:40


Mathematics, 15.12.2020 23:40





Chemistry, 15.12.2020 23:40

Mathematics, 15.12.2020 23:40

Mathematics, 15.12.2020 23:40

Chemistry, 15.12.2020 23:40

Mathematics, 15.12.2020 23:40

English, 15.12.2020 23:40

History, 15.12.2020 23:40

 ...[1]
...[1]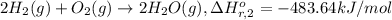 ...[2]
...[2]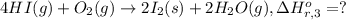 ..[3]
..[3]