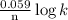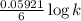Chemistry, 19.10.2019 05:10 bethdove9466
What is the value of the equilibrium constant for the reaction between cr(s) and cu^2+ (aq) at 25 degree c? cr(s) + cu^2+ (q)rightarrow cr^3+ (aq) + cu(s) if your calculator has overflow issues with this problem, you can use this identity to solve for k: if log(k) = x + y then k = 10^x times 10^y 3 times 10^109 -6 times 10^5 2 times 10^18 1 times 10^2 3 times 10^40

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
What is the value of the equilibrium constant for the reaction between cr(s) and cu^2+ (aq) at 25 de...
Questions

Mathematics, 04.04.2020 04:00





English, 04.04.2020 04:00












History, 04.04.2020 04:00

Mathematics, 04.04.2020 04:00