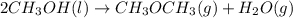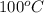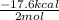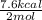Given the standard heat of combustion of methanol ch3oh is 182.6
kcal/mol, dimethyl ether ch3och3 is 347.6 kcal/mol, (methanol/
dimethyl ether in gas phase, water in liquid phase). given the heat
of vaporization of water is 10 kcal/mol, methanol is 8.4 kcal/mol,
dimethyl ether 4.8 kcal/mol. calculate the reaction of dehydration
of methanol to produce dimethyl ether. (indicate the phase of your
components).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1

You know the right answer?
Given the standard heat of combustion of methanol ch3oh is 182.6
kcal/mol, dimethyl ethe...
kcal/mol, dimethyl ethe...
Questions



Spanish, 02.08.2019 05:30




Mathematics, 02.08.2019 05:30







History, 02.08.2019 05:30

History, 02.08.2019 05:30

Mathematics, 02.08.2019 05:30

History, 02.08.2019 05:30

Physics, 02.08.2019 05:30


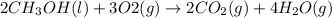 ....... (1)
....... (1)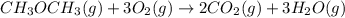 -347.6 kcal/mol
-347.6 kcal/mol
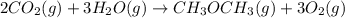 heat of reaction +347.6 kcal {sign reversed with reaction} ........ (2)
heat of reaction +347.6 kcal {sign reversed with reaction} ........ (2)