
Chemistry, 21.11.2019 20:31 ilovewaffles70
Which statement best explains why the polarity of a h2o molecule differs from the polarity of a co2 molecule? the bond strength is greater in co2. oxygen is more electronegative than carbon. the central atom in h2o has lone pair electrons and the central atom in co2 does not.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1
You know the right answer?
Which statement best explains why the polarity of a h2o molecule differs from the polarity of a co2...
Questions


History, 10.12.2019 20:31





Mathematics, 10.12.2019 20:31

History, 10.12.2019 20:31





Spanish, 10.12.2019 20:31

Mathematics, 10.12.2019 20:31






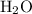 molecule differs from the polarity of
molecule differs from the polarity of 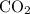 molecule, because Oxygen is more electronegative than carbon.
molecule, because Oxygen is more electronegative than carbon. is 3.44.
is 3.44.

