Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2
You know the right answer?
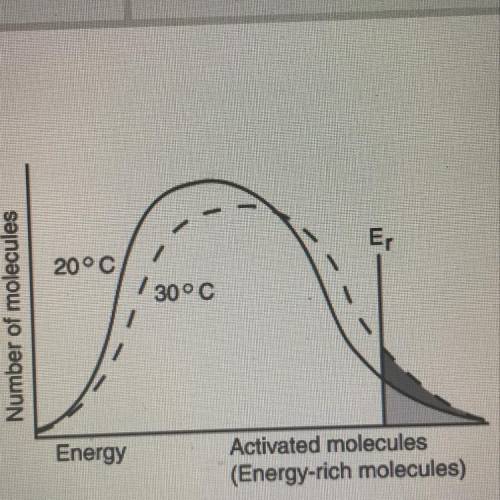
From the diagram, when the temperatures of reactants are raised from 20°C to 30°C:
The average...
The average...
Questions



History, 11.10.2019 10:30

Mathematics, 11.10.2019 10:30


World Languages, 11.10.2019 10:30

Mathematics, 11.10.2019 10:30

English, 11.10.2019 10:30

English, 11.10.2019 10:30


Chemistry, 11.10.2019 10:30


Mathematics, 11.10.2019 10:30



History, 11.10.2019 10:30

Mathematics, 11.10.2019 10:30

Engineering, 11.10.2019 10:30

Biology, 11.10.2019 10:30