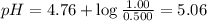Chemistry, 14.02.2020 03:28 andydiaz1227
What is the pH of a solution that is 0.500 M in acetic acid and 1.00 M in CH3COONa? Ka = 1.75*10-5 Group of answer choices 4.47 5.06 4.77 0.3 Flag this Question

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1

Chemistry, 23.06.2019 08:40
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1
You know the right answer?
What is the pH of a solution that is 0.500 M in acetic acid and 1.00 M in CH3COONa? Ka = 1.75*10-5 G...
Questions



History, 07.12.2020 22:00



English, 07.12.2020 22:00


Mathematics, 07.12.2020 22:00



Biology, 07.12.2020 22:00




Computers and Technology, 07.12.2020 22:00

Social Studies, 07.12.2020 22:00



Mathematics, 07.12.2020 22:00