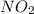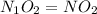Chemistry, 20.02.2020 20:58 ekarlewicz3343
Nitrogen and oxygen form an extensive series of oxides with the general formula NxOy. What is the empirical formula for an oxide that contains 30.44% by mass nitrogen?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Nitrogen and oxygen form an extensive series of oxides with the general formula NxOy. What is the em...
Questions

Mathematics, 27.05.2020 07:58

English, 27.05.2020 07:58



Physics, 27.05.2020 07:58

Mathematics, 27.05.2020 07:58

English, 27.05.2020 07:58

History, 27.05.2020 07:58

Chemistry, 27.05.2020 07:58

Mathematics, 27.05.2020 07:58


Social Studies, 27.05.2020 07:58


Mathematics, 27.05.2020 07:58

Health, 27.05.2020 07:58

Biology, 27.05.2020 07:58

English, 27.05.2020 07:59