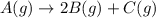Chemistry, 21.02.2020 01:15 ToonGamesToo
Consider the generic reaction: A (g) → 2 B (g) + C (g) . If a flask initially contains only A and the reaction proceeds to completion with a final pressure of 38.9 atm , what is the partial pressure of B in the container? (Assume constant volume and temperature.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
Consider the generic reaction: A (g) → 2 B (g) + C (g) . If a flask initially contains only A and th...
Questions

Mathematics, 13.12.2020 06:30

Mathematics, 13.12.2020 06:30


Mathematics, 13.12.2020 06:30


Spanish, 13.12.2020 06:30

Mathematics, 13.12.2020 06:30

Computers and Technology, 13.12.2020 06:30





Mathematics, 13.12.2020 06:30


Mathematics, 13.12.2020 06:30

English, 13.12.2020 06:30



Business, 13.12.2020 06:30

Business, 13.12.2020 06:30