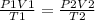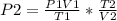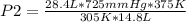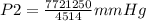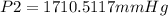Chemistry, 22.02.2020 02:28 jaksmmwlqlzm
A sample of gas with an initial volume of 28.4 Liters at a pressure of 725 mmHg and a temperature of 305 K is compressed to a volume of 14.8 Liters and warmed to a temperature of 375 Kelvin. What is the final pressure of the gas?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3


You know the right answer?
A sample of gas with an initial volume of 28.4 Liters at a pressure of 725 mmHg and a temperature of...
Questions

Mathematics, 23.11.2020 21:10


Social Studies, 23.11.2020 21:10

Mathematics, 23.11.2020 21:10


Spanish, 23.11.2020 21:10

Advanced Placement (AP), 23.11.2020 21:10

Biology, 23.11.2020 21:10




Biology, 23.11.2020 21:10

Mathematics, 23.11.2020 21:10

English, 23.11.2020 21:10

Mathematics, 23.11.2020 21:10


English, 23.11.2020 21:10