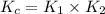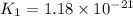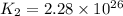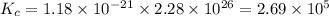Consider the following reactions and their equilibrium constants at 303 K. PCl5(g) equilibrium reaction arrow 1/4 P4(g) + 5/2 Cl2(g); Kc = 1.18 ✕ 10−21 1/4 P4(g) + 3/2 Cl2(g) equilibrium reaction arrow PCl3(g); Kc = 2.28 ✕ 1026 Calculate Kc for PCl5(g) equilibrium reaction arrow PCl3(g) + Cl2(g) at the same temperature.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
Consider the following reactions and their equilibrium constants at 303 K. PCl5(g) equilibrium react...
Questions

Arts, 27.10.2019 12:43

History, 27.10.2019 12:43


French, 27.10.2019 12:43


Mathematics, 27.10.2019 12:43








History, 27.10.2019 12:43

Business, 27.10.2019 12:43




Biology, 27.10.2019 12:43

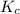 for the net reaction is
for the net reaction is 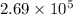
![PCl_5(g)\xrightarrow[]{K_1} \frac{1}{4}P_4(g)+\frac{5}{2}Cl_2(g)](/tpl/images/0537/3407/f38c5.png)
![\frac{1}{4}P_4(g)+\frac{3}{2}Cl_2(g)\xrightarrow[]{K_2} PCl_5(g)](/tpl/images/0537/3407/948a1.png)
![PCl_5(g)\xrightarrow[]{K_c} PCl_3(g)+Cl_2(g)](/tpl/images/0537/3407/3c32f.png)