
Chemistry, 11.03.2020 13:33 lildanielmabien
Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 react with excess HCl according to the following equation.
Al2O3(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2O(l)
Group of answer choices
72.9 g
155 g
65.4 g
16.3 g
32.6 g

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 react with excess HCl according to...
Questions


Mathematics, 10.07.2019 19:00





Biology, 10.07.2019 19:00


Mathematics, 10.07.2019 19:00




Mathematics, 10.07.2019 19:00

Mathematics, 10.07.2019 19:00

Social Studies, 10.07.2019 19:00

Mathematics, 10.07.2019 19:00

Chemistry, 10.07.2019 19:00



Mathematics, 10.07.2019 19:00

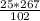 = 65.4 g of AlCl₃.
= 65.4 g of AlCl₃.

