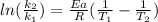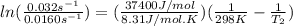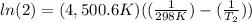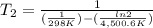Chemistry, 12.03.2020 20:03 shelbylynn17
The activation energy of a certain reaction is 37.4 kJ/mol . At 25 ∘C , the rate constant is 0.0160s−1 . At what temperature in degrees Celsius would this reaction go twice as fast?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1
You know the right answer?
The activation energy of a certain reaction is 37.4 kJ/mol . At 25 ∘C , the rate constant is 0.0160s...
Questions



Mathematics, 17.04.2020 16:20









Mathematics, 17.04.2020 16:20



Biology, 17.04.2020 16:20



Physics, 17.04.2020 16:20

Mathematics, 17.04.2020 16:20