
Chemistry, 16.03.2020 22:34 McGregorshamara
Write the dissolution reaction for iron(III) nitrate in water. Use the pull-down menus to specify the state of each reactant and product. + Is iron(III) nitrate considered soluble or not soluble ? ... ... A. Soluble ... B. Not soluble Based upon this, the equilibrium constant for this reaction will be: ... ... A. Greater than 1 ... B. Less than 1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Write the dissolution reaction for iron(III) nitrate in water. Use the pull-down menus to specify th...
Questions




Mathematics, 30.01.2020 23:56


History, 30.01.2020 23:56


Social Studies, 30.01.2020 23:56


World Languages, 30.01.2020 23:56

History, 30.01.2020 23:56

Physics, 30.01.2020 23:56

History, 30.01.2020 23:56

Chemistry, 30.01.2020 23:56


SAT, 30.01.2020 23:56



History, 30.01.2020 23:56

Geography, 30.01.2020 23:56

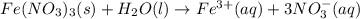
![K_{eq} = [Fe^{3+}][(NO_{3})_{3}]^{3}](/tpl/images/0549/4240/1e6b7.png)


