
Chemistry, 19.03.2020 19:26 austinmontgomep7foxp
The following two reactions are important in the blast furnace production of iron metal from iron ore (Fe2O3):2C(s) + O2(g) -> 2CO(g)Fe2O3 + 3CO(g) -> 2Fe + 3CO2Using these balanced reactions, how many moles of O2 are required for the production of 5.00 kg of Fe?A) 67.1 molesB) 29.8 molesC) 7.46 molesD) 89.5 molesE) 16.8 moles

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2


Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1
You know the right answer?
The following two reactions are important in the blast furnace production of iron metal from iron or...
Questions

Biology, 25.05.2020 21:57

Spanish, 25.05.2020 21:57

Mathematics, 25.05.2020 21:57

Mathematics, 25.05.2020 21:57


Mathematics, 25.05.2020 21:57


Mathematics, 25.05.2020 21:57



History, 25.05.2020 21:57

English, 25.05.2020 21:57

Mathematics, 25.05.2020 21:57

English, 25.05.2020 21:57

Chemistry, 25.05.2020 21:57


Mathematics, 25.05.2020 21:57


Mathematics, 25.05.2020 21:57

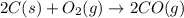
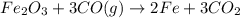
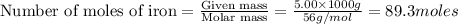
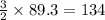 moles of carbon monoxide
moles of carbon monoxide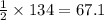 moles of oxygen
moles of oxygen

