
Chemistry, 19.03.2020 22:34 skatingflower
When the chemical reaction A + B ⇀↽ C + D is at equilibrium, 1. neither the forward nor the reverse reactions have stopped. 2. all four concentrations are equal. 3. both the forward and reverse reactions have stopped. 4. the sum of the concentrations of A and B equals the sum of the concentrations of C and D. 5. the reverse reaction has stopped. 6. the forward reaction has stopped.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
When the chemical reaction A + B ⇀↽ C + D is at equilibrium, 1. neither the forward nor the reverse...
Questions







Computers and Technology, 16.10.2020 02:01

History, 16.10.2020 02:01

Mathematics, 16.10.2020 02:01

Geography, 16.10.2020 02:01



History, 16.10.2020 02:01







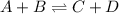
![K=\frac{[C]\times [D]}{[A]\times [B}}](/tpl/images/0554/7763/ae12b.png)


