
Chemistry, 19.03.2020 23:13 esmeralda266
Chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 250.mL sample of groundwater known to be contaminated with iron(III) chloride, which would react with silver nitrate solution like this:FeCl3(aq) + 3AgNO3(aq) ⟶ 3AgCl(s) + FeNO3(aq)The chemist adds 82.0 M silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected 2.5mg of silver chloride. Calculate the concentration of iron(III) chloride contaminant in the original groundwater sample.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 19:30
Atax that increases in proportion to increase in income is known as
Answers: 1

Chemistry, 24.06.2019 00:40
What two factors speed up rates of chemical reaction and weathering in rocks and soils?
Answers: 1
You know the right answer?
Chloride anions in solution will combine with the silver cations to produce bright white silver chlo...
Questions



Mathematics, 17.03.2020 00:55









Mathematics, 17.03.2020 00:55







Mathematics, 17.03.2020 00:55

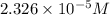 the concentration of iron(III) chloride contaminant in the original groundwater sample.
the concentration of iron(III) chloride contaminant in the original groundwater sample.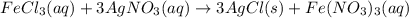
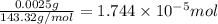
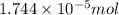 od silver chloride will be obtained from ;
od silver chloride will be obtained from ;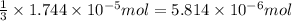 of ferric chloride
of ferric chloride![[FeCl_3]=\frac{5.814\times 10^{-6} mol}{0.250 L}=2.326\times 10^{-5} M](/tpl/images/0554/8189/91194.png)


