
Chemistry, 24.03.2020 19:49 Lovelybunny321
Methylamine, CH3NH2, is a weak base that reacts according to the reaction CH3NH2 + H2O <--> CH3NH3+ + OH- The value of the ionization constant, Kb, is 5.25 x 10 –4. Methylamine reacts to form salts such as methylammonium nitrate, (CH3NH3+)(NO3-). a. Calculate the hydroxide ion concentration, [OH-] of a 0.125 molar aqueous solution of methylamine.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Methylamine, CH3NH2, is a weak base that reacts according to the reaction CH3NH2 + H2O <--> CH...
Questions

Mathematics, 20.09.2020 03:01


Computers and Technology, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01






Mathematics, 20.09.2020 03:01


History, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01

History, 20.09.2020 03:01

Computers and Technology, 20.09.2020 03:01

Biology, 20.09.2020 03:01

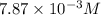
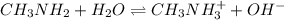
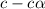
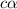
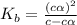
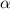 = ?
= ?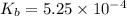
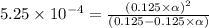
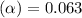
![[OH^-]=c\times \alpha](/tpl/images/0561/1862/0ea5e.png)
![[OH^-]=0.125\times 0.063=7.87\times 10^{-3}M](/tpl/images/0561/1862/08078.png)


