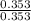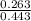A buffer solution is made that is 0.353 M in CH3COOH and 0.353 M in CH3COO- . (1) If Ka for CH3COOH is 1.80×10-5 , what is the pH of the buffer solution? (2) Write the net ionic equation for the reaction that occurs when 0.090 mol HI is added to 1.00 L of the buffer solution. Use H3O+ instead of H+ .

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
We just started a new lesson in chemistry and everyone hates it and i dont get it one bit. i hate school. h el p.balanced equationc3h8+5o2-> 3co2+4h2o1.) if you start with 14.8g of propane(c3h8) and 3.44g of oxygen, which is the limiting reactant -check my answer 2.)what mass of excess reagent is left over? 3.)what mass of carbon dioxide can be made? 4.)what mass of water is produced?
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
A buffer solution is made that is 0.353 M in CH3COOH and 0.353 M in CH3COO- . (1) If Ka for CH3COOH...
Questions

Mathematics, 05.02.2020 04:49

History, 05.02.2020 04:49


Mathematics, 05.02.2020 04:49




Computers and Technology, 05.02.2020 04:49


Mathematics, 05.02.2020 04:49


History, 05.02.2020 04:49

Computers and Technology, 05.02.2020 04:49

Geography, 05.02.2020 04:49






![\frac{[Salt]}{[Acid]}](/tpl/images/0561/5667/6743a.png)