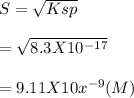Chemistry, 07.04.2020 21:33 skylar1315
Calculate the molar solubility of AgI in the following. Ksp for AgI is 8.30 ×10−17.(a) pure water×10MEnter your answer in scientific notation.(b) 0.0400 M NaI×10MEnter your answer in scientific notation.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3



You know the right answer?
Calculate the molar solubility of AgI in the following. Ksp for AgI is 8.30 ×10−17.(a) pure water×10...
Questions

Mathematics, 06.04.2021 18:40






Mathematics, 06.04.2021 18:40


Social Studies, 06.04.2021 18:40


English, 06.04.2021 18:40


History, 06.04.2021 18:40

Mathematics, 06.04.2021 18:40