Chemistry, 16.04.2020 00:23 lerasteidl
Measurements show that the enthalpy of a mixture of gaseous reactants increases by 243.kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that −174.kJ of work is done on the mixture during the reaction. Calculate the change of energy of the gas mixture during the reaction in kJ.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
Measurements show that the enthalpy of a mixture of gaseous reactants increases by 243.kJ during a c...
Questions





Computers and Technology, 10.11.2020 06:10




World Languages, 10.11.2020 06:10


English, 10.11.2020 06:10


History, 10.11.2020 06:10




Mathematics, 10.11.2020 06:10

English, 10.11.2020 06:10


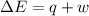
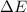 =Change in internal energy
=Change in internal energy