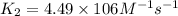Chemistry, 21.04.2020 18:50 xxaurorabluexx
For the second-order reaction NO( g) + O 3( g) → NO 2( g) + O 2( g), the rate constant has been measured to be 1.08 × 10 7 M –1 s –1 at 298 K and the activation energy has been measured to be 11.4 kJ/mol over the temperature range 195 K to 304 K. What is the rate constant at 207 K? ( R = 8.3145 J K –1 mol –1)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3
You know the right answer?
For the second-order reaction NO( g) + O 3( g) → NO 2( g) + O 2( g), the rate constant has been meas...
Questions







Geography, 26.07.2019 06:30

Health, 26.07.2019 06:30

Biology, 26.07.2019 06:30

Chemistry, 26.07.2019 06:30




Advanced Placement (AP), 26.07.2019 06:30

Chemistry, 26.07.2019 06:30




Chemistry, 26.07.2019 06:30

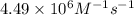
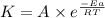
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0615/2426/6d953.png)
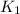 = rate constant at
= rate constant at 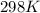 =
= 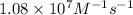
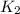 = rate constant at
= rate constant at 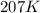 = ?
= ?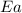 = activation energy for the reaction =
= activation energy for the reaction = 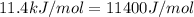
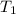 = initial temperature = 298 K
= initial temperature = 298 K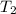 = final temperature = 207 K
= final temperature = 207 K![\log (\frac{K_2}{1.08\times 10^7M^{-1}s^{-1}})=\frac{11400J/mol}{2.303\times 8.314J/mole.K}[\frac{1}{298K}-\frac{1}{207K}]](/tpl/images/0615/2426/e96a1.png)