Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1



Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
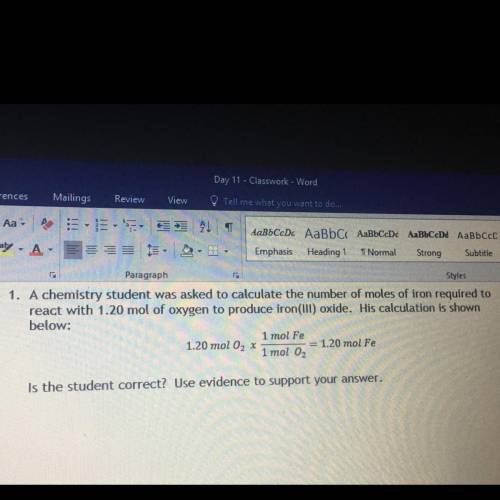
A chemistry student was asked to calculate the number of moles of iron required to react with 1.20 m...
Questions



Biology, 18.09.2019 02:30

Geography, 18.09.2019 02:30



Social Studies, 18.09.2019 02:30

Physics, 18.09.2019 02:30

Health, 18.09.2019 02:30

History, 18.09.2019 02:30

Mathematics, 18.09.2019 02:30



Business, 18.09.2019 02:30




Social Studies, 18.09.2019 02:30

Advanced Placement (AP), 18.09.2019 02:30