
Chemistry, 25.04.2020 23:51 gabrielolivas59
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O (l)
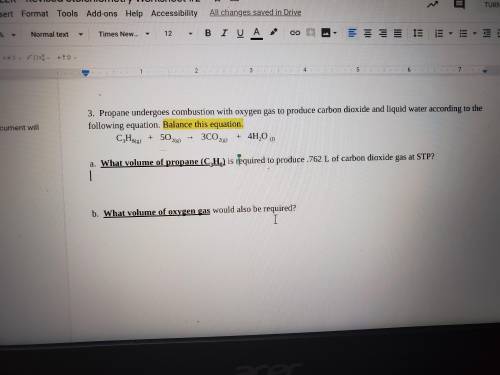
a. What volume of propane (C3H8) is required to produce .762 L of carbon dioxide gas at STP?
b. What volume of oxygen gas would also be required?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O (l)
a. What volume of propane (C3H8) is required to pr...
a. What volume of propane (C3H8) is required to pr...
Questions


Biology, 31.01.2020 20:43





Mathematics, 31.01.2020 20:43


History, 31.01.2020 20:43

Physics, 31.01.2020 20:43




Biology, 31.01.2020 20:43

Mathematics, 31.01.2020 20:43

History, 31.01.2020 20:43

Mathematics, 31.01.2020 20:43

Mathematics, 31.01.2020 20:43


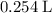 (at STP.)
(at STP.)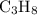 and
and 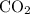 act like ideal gases.
act like ideal gases.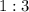 . As a result, for every
. As a result, for every 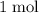 of
of 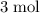 of
of 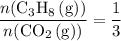 .
.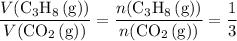 .
.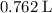 of
of 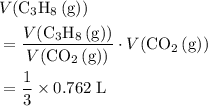 .
.

