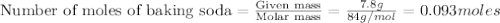Chemistry, 10.06.2020 06:57 smithsa10630
1.)A strong acid solution requires 3.2 grams of sulfuric acid (H2SO4). How many molecules of sulfuric acid are in the solution? 2.) While measuring out the sulfuric acid you accidentally spilled some of it! Before trying to clean it up you put some baking soda (NaHCO3) on to it neutralize it. If you scatter 7.8 g of baking soda on the acid how many moles of baking soda have you used?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 13:00
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2

Chemistry, 23.06.2019 16:30
There is a set up transformer that doubles the voltage. if the primary coil has a voltage of 10 v
Answers: 2
You know the right answer?
1.)A strong acid solution requires 3.2 grams of sulfuric acid (H2SO4). How many molecules of sulfuri...
Questions




Mathematics, 23.02.2021 22:10


Mathematics, 23.02.2021 22:10


Mathematics, 23.02.2021 22:10

Mathematics, 23.02.2021 22:10


Mathematics, 23.02.2021 22:10

SAT, 23.02.2021 22:10

Mathematics, 23.02.2021 22:10

English, 23.02.2021 22:10

Mathematics, 23.02.2021 22:10



Social Studies, 23.02.2021 22:10

History, 23.02.2021 22:10

Mathematics, 23.02.2021 22:10

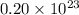 molecules of sulfuric acid in the solution.
molecules of sulfuric acid in the solution.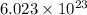 of particles.
of particles.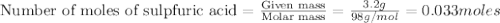
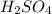 contains =
contains = 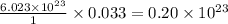 molecules of sulfuric acid
molecules of sulfuric acid