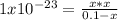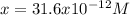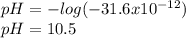Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
consider an exceptionally weak acid, HA, with Ka= 1 x 10-20. you make 0.1M solution of the salt NA....
Questions

Mathematics, 24.11.2020 23:20


Social Studies, 24.11.2020 23:20



Mathematics, 24.11.2020 23:20

Biology, 24.11.2020 23:30

Mathematics, 24.11.2020 23:30

Biology, 24.11.2020 23:30






History, 24.11.2020 23:30



Mathematics, 24.11.2020 23:30


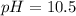
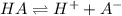
![Ka=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0682/3092/39962.png)
 due to the reaction extent is:
due to the reaction extent is: