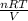Chemistry, 12.11.2020 22:50 footboy773
Si tuvieras que averiguar la presión a la que se encuentran 5 moles un gas contenido en un recipiente de 3 litros a 290K ¿Que ecuación emplearías?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
Si tuvieras que averiguar la presión a la que se encuentran 5 moles un gas contenido en un recipient...
Questions

Mathematics, 09.01.2021 20:40

German, 09.01.2021 20:40

English, 09.01.2021 20:40


Social Studies, 09.01.2021 20:40

Mathematics, 09.01.2021 20:40






Mathematics, 09.01.2021 20:50





Arts, 09.01.2021 20:50

Arts, 09.01.2021 20:50