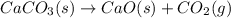Chemistry, 18.11.2020 17:10 julianbeaver76
Solid calcium carbonate decomposes to produce solid calcium oxide and carbon dioxide gas. Express your answer as a chemical equation. Identify all of the phases in your answer. CaCO,(s)-CaO(s) + CO2(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1
You know the right answer?
Solid calcium carbonate decomposes to produce solid calcium oxide and carbon dioxide gas. Express yo...
Questions


Mathematics, 01.11.2019 23:31

Mathematics, 01.11.2019 23:31

Mathematics, 01.11.2019 23:31





History, 01.11.2019 23:31


Mathematics, 01.11.2019 23:31






Mathematics, 01.11.2019 23:31


Biology, 01.11.2019 23:31

History, 01.11.2019 23:31