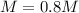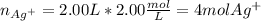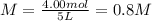Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

You know the right answer?
You have 3.00 L of a 3.12 M solution of NaCl(aq) called solution A. You also have 2.00 L of a 2.00 M...
Questions

Computers and Technology, 23.06.2021 14:00

Computers and Technology, 23.06.2021 14:00



Physics, 23.06.2021 14:00

Social Studies, 23.06.2021 14:00

Computers and Technology, 23.06.2021 14:00

Mathematics, 23.06.2021 14:00

Mathematics, 23.06.2021 14:00

Mathematics, 23.06.2021 14:00

English, 23.06.2021 14:00

Physics, 23.06.2021 14:00



Geography, 23.06.2021 14:00

Mathematics, 23.06.2021 14:00

Business, 23.06.2021 14:00



Mathematics, 23.06.2021 14:00