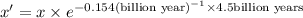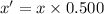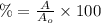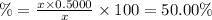Chemistry, 18.08.2019 15:20 wafflewarriormg
Uranium-238 has a half-life of 4.5 billion years. given that scientists estimate earth's age to be 4.6 billion years, what is the most likely percentage of parent to daughter isotopes of this element currently existing on earth? a. 10 percent b. 25 percent c. 50 percent d. 75 percent

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
Uranium-238 has a half-life of 4.5 billion years. given that scientists estimate earth's age to be 4...
Questions

Mathematics, 05.10.2021 19:20


Engineering, 05.10.2021 19:20


Biology, 05.10.2021 19:20

Physics, 05.10.2021 19:20

Mathematics, 05.10.2021 19:20







Computers and Technology, 05.10.2021 19:20

Mathematics, 05.10.2021 19:20


Biology, 05.10.2021 19:20


Mathematics, 05.10.2021 19:20

Mathematics, 05.10.2021 19:20

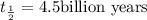
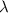
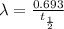
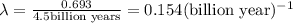
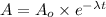
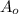 = Initial amount
= Initial amount