
Chemistry, 06.03.2021 01:00 randyg0531
Fe2O3 + 3 CO = 2Fe + 3 CO2
In the reaction above, how many grams of Fe2O3 are required to completely react with 63.78 grams of CO?
a.121.2 g
b. 23.0 g
c 80.4 g
d. 110 g
*refer to attachment*
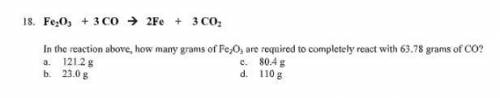

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 23.06.2019 10:30
Amethod of separation that employs a system with two phases of matter, a mobile phase and a stationary phase, is called
Answers: 2

Chemistry, 23.06.2019 12:20
Amatch has about 21 milligrams of red phosphorus coating the tip. how many atoms of phosphorus is this?
Answers: 1
You know the right answer?
Fe2O3 + 3 CO = 2Fe + 3 CO2
In the reaction above, how many grams of Fe2O3 are required to completel...
Questions


Mathematics, 16.10.2019 20:00


Geography, 16.10.2019 20:00

Health, 16.10.2019 20:00





English, 16.10.2019 20:00





History, 16.10.2019 20:00

Spanish, 16.10.2019 20:00



History, 16.10.2019 20:00

History, 16.10.2019 20:00



