
Chemistry, 11.03.2021 20:00 07corcum85504
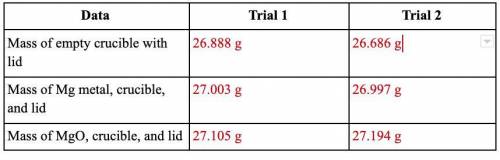
40 Points! Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
Equation: Mg + O2 → MgO
This is the actual yield of magnesium oxide for each trial.
Trial 1: 0.217 g
Trial 2: 0.508 g

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
40 Points! Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of...
Questions

Mathematics, 22.04.2020 18:53


Mathematics, 22.04.2020 18:53

History, 22.04.2020 18:53


Mathematics, 22.04.2020 18:53

History, 22.04.2020 18:53

Mathematics, 22.04.2020 18:53

Physics, 22.04.2020 18:53

Mathematics, 22.04.2020 18:53

Health, 22.04.2020 18:53

Mathematics, 22.04.2020 18:53

Mathematics, 22.04.2020 18:53



Computers and Technology, 22.04.2020 18:53

Social Studies, 22.04.2020 18:53

Business, 22.04.2020 18:53




