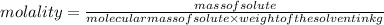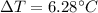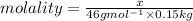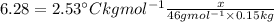Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
What mass of solid that has a molar mass of 46.0 g/mol should be added to 150.0 g of benzene to rais...
Questions


Chemistry, 29.08.2021 09:10


Mathematics, 29.08.2021 09:10

History, 29.08.2021 09:20

Health, 29.08.2021 09:20

Biology, 29.08.2021 09:20

Physics, 29.08.2021 09:20




Physics, 29.08.2021 09:20



Mathematics, 29.08.2021 09:20

English, 29.08.2021 09:20

Mathematics, 29.08.2021 09:20



History, 29.08.2021 09:20

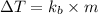
 = elevation in boiling point
= elevation in boiling point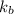 = boiling point elevation constant
= boiling point elevation constant