

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
You know the right answer?
For the reaction, calculate how many moles of the product form when 0.048 mol of o2 completely react...
Questions

English, 19.10.2021 14:00

Social Studies, 19.10.2021 14:00


Business, 19.10.2021 14:00



Mathematics, 19.10.2021 14:00

Spanish, 19.10.2021 14:00

Mathematics, 19.10.2021 14:00


Mathematics, 19.10.2021 14:00

Mathematics, 19.10.2021 14:00

English, 19.10.2021 14:00

Mathematics, 19.10.2021 14:00




Mathematics, 19.10.2021 14:00


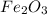 form are 0.032 moles.
form are 0.032 moles.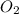 = 0.048 mole
= 0.048 mole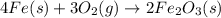
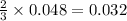 moles of
moles of 

