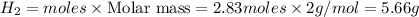Work the entire problem from the beginning. how many grams of h2 would be formed if 34 grams of carbon reacted with an unlimited amount of h2o? the reaction is: c + h2o → co + h2 the atomic mass of c is 12.01 g/mole. the atomic mass of h2 is 2.016 g/mole. grams of hydrogen

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
Work the entire problem from the beginning. how many grams of h2 would be formed if 34 grams of carb...
Questions

Mathematics, 12.06.2020 23:57

Mathematics, 12.06.2020 23:57

History, 12.06.2020 23:57

Mathematics, 12.06.2020 23:57


Mathematics, 12.06.2020 23:57


Chemistry, 12.06.2020 23:57

Chemistry, 12.06.2020 23:57


Mathematics, 12.06.2020 23:57


English, 12.06.2020 23:57

Mathematics, 12.06.2020 23:57

Mathematics, 12.06.2020 23:57

Mathematics, 12.06.2020 23:57



History, 12.06.2020 23:57

Mathematics, 12.06.2020 23:57

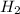 would be formed if 34 grams of carbon reacted with an unlimited amount of
would be formed if 34 grams of carbon reacted with an unlimited amount of 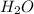
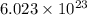 of particles.
of particles.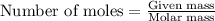
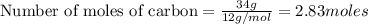
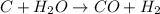
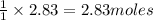 of
of